
Background: Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening hyperinflammatory syndrome that results in histiocyte proliferation and extensive hemophagocytosis. HLH is classified as primary, which is caused by genetic defects, or secondary, which occurs secondary to infections, malignancies, rheumatologic diseases or other causes. Both types of HLH are rare and have a nonspecific clinical presentation which makes the diagnosis challenging. The diagnosis and treatment of most HLH cases are guided by protocols developed by the Histiocyte Society (HLH-94 and -2004), which are based on data from pediatric populations. There is limited data regarding treatment and outcomes of HLH in adults. In this study, we reviewed the patients with HLH at 2 academic tertiary care centers in 2 different states.
Methods: We retrospectively reviewed patients who had an ICD-10 code of D76.1, corresponding to diagnosis of HLH or macrophage activation syndrome (MAS) at University of Nebraska Medical Center (UNMC) or University of Kentucky Medical Center (UKMC) between January 2008-April 2020. Only patients who were >18 years of age and diagnosed as inpatient where included. Data were collected to assess demographics, etiology, symptoms, diagnostic criteria, H-score, treatment, systemic complications, and overall survival (OS). The Kaplan-Meier method was used to estimate OS and log-rank test was used for comparisons.
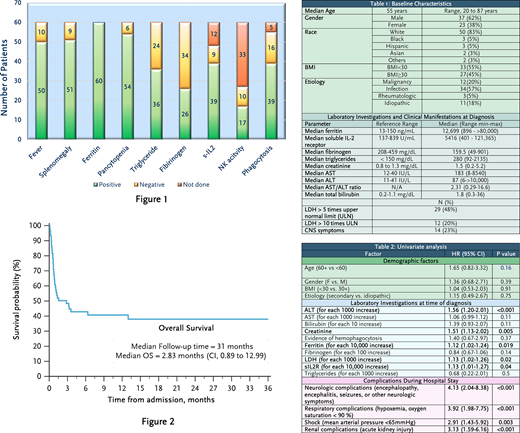
Results: A total of 60 patients were identified (27 from UNMC and 33 from UKMC) with a mean age of 55 years (20-87 years). Fifty-two (87%) patients met at least 5 of the HLH 2004 criteria. The median H-score was 241 (119-322). Elevated ferritin was the most common criterion that was met (100%), followed by cytopenias (Figure 1). Median ferritin and soluble IL2 receptor (sIL2R) were approximately 12,699.5 ng/mL (896 to >80,000 ng/ml) and 5416 U/mL (401-121,365 pg/mL), respectively. Most patients had elevated creatinine, hypofibrinogenemia, hyperbilirubinemia and/or significant transaminitis with a median AST/ALT ratio of > 2. Table 1 summarizes demographics, etiologies and laboratory investigations at time of diagnosis. Fifty-one percent of patients were treated based on HLH protocols, 12% received glucocorticoids only, 5% received therapy directed against their malignancy, and the rest received other treatments. Twenty-six (43%) patients achieved complete response, 7 patients relapsed (3 achieved completed response with second treatment, 4 died [one of them after a third relapse]) and 27 patients (45%) died. After a median follow-up of 31 months, there were 35 deaths (58%), with median OS of 2.83 months (CI, 0.89 to 12.99) (Figure 2). At least 16 (27%) patients were alive after one year follow up. Among patients who succumbed to HLH, the median time from admission until death was 21.5 days (4-395 days) (95% CI, 17.5-28.5). On univariate analysis, increased ALT, creatinine, ferritin, LDH and sIL2R at the time of diagnosis were associated with worse OS (Table 2). In addition, patients who developed shock, renal impairment, respiratory or neurologic complications also had worse outcomes. On the other hand, age, sex, BMI, primary vs. secondary etiology, AST, bilirubin, fibrinogen, triglycerides, and absence of hemophagocytosis on tissue were not associated with survival. Only 41 patients (68%) had available data for all relevant predictive variables, which did not allow for full multivariate analysis.
Conclusion: HLH is a rare and life-threatening syndrome. It is associated with high mortality often observed within the 30 days of diagnosis. Poor prognostic factors in our cohort included elevated levels of ferritin, ALT, LDH, creatinine and/or sIL2R at the time of diagnosis as well as subsequent the development of shock, renal, respiratory, and/or neurologic complications during hospital stay. If verified in a prospective manner, these predictors of adverse outcomes may help further refine risk-stratification of adult HLH patients at diagnosis.
Armitage:Samus Therapeutics: Consultancy; Ascentage: Consultancy; Trovagene/Cardiff Oncology: Membership on an entity's Board of Directors or advisory committees. Vose:Janssen: Honoraria; Incyte: Research Funding; AbbVie: Consultancy, Honoraria; Epizyme: Honoraria, Research Funding; Wugen: Honoraria; Bristol-Myers Squibb: Research Funding; Celgene: Honoraria; Roche/Genetech: Consultancy, Honoraria, Other; Seattle Genetics: Research Funding; Novartis: Research Funding; Loxo: Consultancy, Honoraria, Research Funding; Miltenyi Biotec: Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; Verastem: Consultancy, Honoraria; Karyopharm Therapeutics: Consultancy, Honoraria; Allogene: Honoraria; Kite, a Gilead Company: Honoraria, Research Funding. Hildebrandt:Charlottes Webb CWBHF: Other; CVS Health: Other: Stock and Other Ownership Interests ; Axim Biotechnologies: Other: Stock and Other Ownership Interests ; Novartis: Other: Stock and Other Ownership Interests ; Insys Therapeutics: Other: Stock and Other Ownership Interests ; Abbvie: Other: Stock and Other Ownership Interests ; GW Pharmaceuticals: Other: Stock and Other Ownership Interests ; Cardinal Health: Other: Stock and Other Ownership Interests ; Clovis Oncology: Other: Stock and Other Ownership Interests ; Cellectis: Other: Stock and Other Ownership Interests ; Almmune Therapeutics Inc AIMT: Other; Celgene: Other: Stock and Other Ownership Interests ; Bluebird Bio: Other; Bristol-Myers Squibb/Medarex: Other; crispr therapeutics: Other; IDEXX Laboratories: Other; Johnson & Johnson: Other; Pfizer: Other; Procter & Gamble: Other; Vertex: Other; Bayer: Other; Scotts-Miracle: Other; Medical PPTYS TR Inc. MPW: Other; Caretrust Reit Inc CTRE: Other; ANGI Homeservices Inc ANGI: Other; MPW (medical PPTYS): Other; Caretrust (CTRE): Other; Aimmune: Other; Incyte: Consultancy; Takeda: Research Funding; Jazz Pharmaceuticals: Research Funding; Pharmacyclics: Research Funding; Incyte: Research Funding; Astellas Pharma: Research Funding; Falk Foundation: Other; Incyte: Other; Takeda: Other. Baljevic:Amgen: Research Funding; Exelixis: Research Funding; Celgene Corporation / BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Cardinal Health: Consultancy, Membership on an entity's Board of Directors or advisory committees; Putnam Associates: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gerson Lehrman Group: Consultancy, Membership on an entity's Board of Directors or advisory committees; AlphaSights: Consultancy, Membership on an entity's Board of Directors or advisory committees; Coleman: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics Inc.: Honoraria; NCCN Hematologic Malignancies Congress: Honoraria; MediCom Myeloma CME: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal